BioMan: Enzymatic!
Play the Enzymatic! game to learn about how enzymes work.

Enzymes are special proteins that are critical in many chemical reactions that occur in living organisms. Knowing how enzymes work is necessary for understanding how metabolic processes are controlled and maintained within cells. This has broader applications in agriculture, medicine and even in textiles. Use this resource to learn about the structure and function of enzymes.
Enzymes are proteins that act as catalysts; they speed up chemical reactions by providing another pathway for the reaction to occur that requires less energy. This makes processes occur more efficiently.
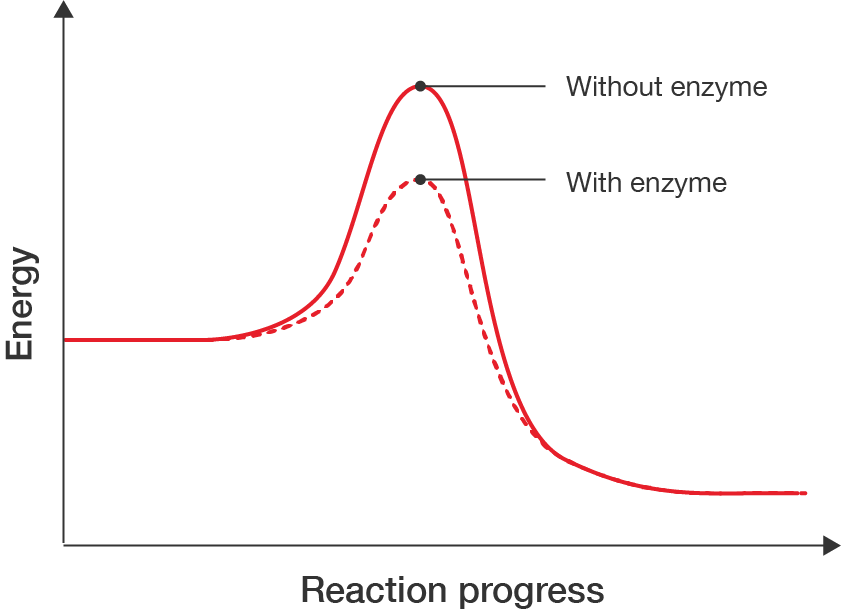
Enzymes play critical roles in a huge range of cellular functions, like digestion, energy production, protein synthesis and DNA replication. Examples of enzymes include RNA polymerase which catalyses the transcription of DNA into RNA, amylase which breaks down complex sugars from your diet into simple sugars that your body can use, and lactase which catalyses the digestion of lactose found in dairy foods.
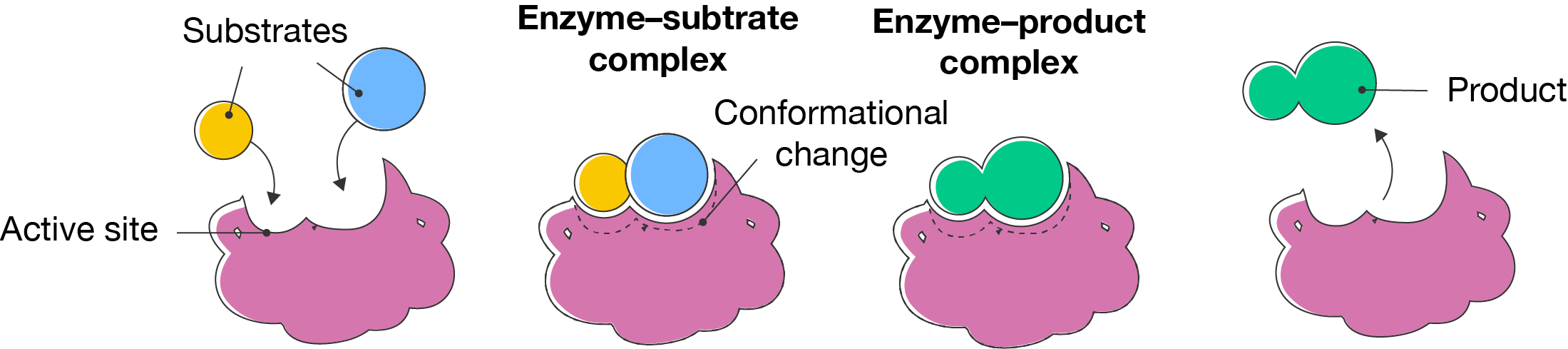
Usually, enzymes work by bringing together two or more molecules, called substrates and joining them, or breaking one substrate into multiple products. Their ability to do this depends on their structure. The site where the enzyme binds the substrates, called the active site needs to the correct shape to bind the substrates.
Once the substrates bind to the enzyme, they form an enzyme–substrate complex. The reaction occurs to form an enzyme–products complex, then the products are released and the enzyme is free to catalyse the next reaction. Enzymes are not consumed in catalysis.
The function of enzymes is described by two models: the lock-and-key model and the induced fit model.
The lock-and-key model of enzyme action was proposed by German chemist Emil Fisher in 1984. He theorised that the shape of an enzyme’s active site was rigid and specifically matched the shape of its substrates, just like a lock has a unique key.

The induced fit model of enzyme action was proposed by American biochemist Daniel E. Koshland in 1958. It is an extension of Fisher’s lock-and-key model, where the enzyme changes shape slightly (or undergoes a conformation change) to bind substrates. This model helped to explain how enzymes can bind more than one type of substrate.
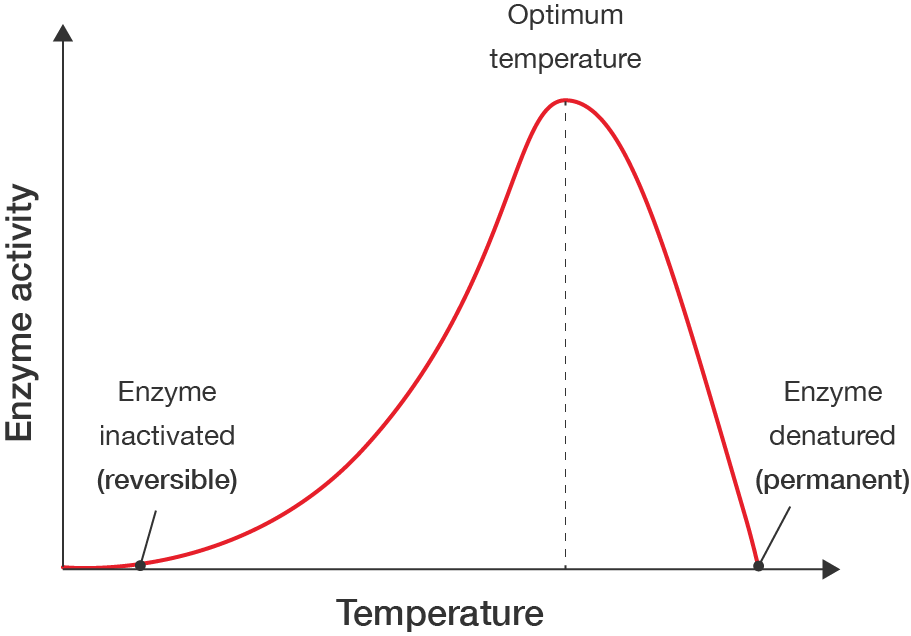
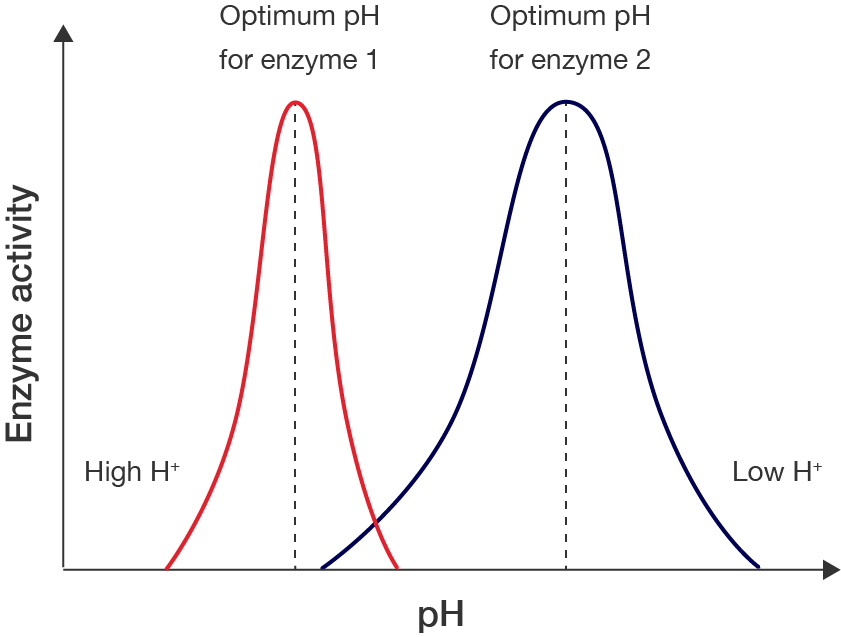
Several factors can affect enzyme action or activity, which then affects the efficiency of chemical reactions. These include:
Enzymes have an optimal temperature range in which they operate best. Below this temperature range, enzymes activity is reduced. At very low temperatures, enzymes can even become inactive. Above this temperature range, enzymes can become denatured. Denaturation involves a permanent change to the enzyme’s structure, causing it to lose its ability to catalyse reactions. Even if the temperature is reduced following denaturation, enzyme activity cannot be recovered, as the shape of the enzyme has changed irreversibly.
Just like temperature, the pH level of the environment in which the enzyme is present affects its activity. This is because enzymes commonly contain acidic and basic groups, which change the electric charge on an enzyme depending on pH.
In some cases, enzymes may rely on their charges to bind with substrates, thus the absence of these charges may affect their ability to perform their function.
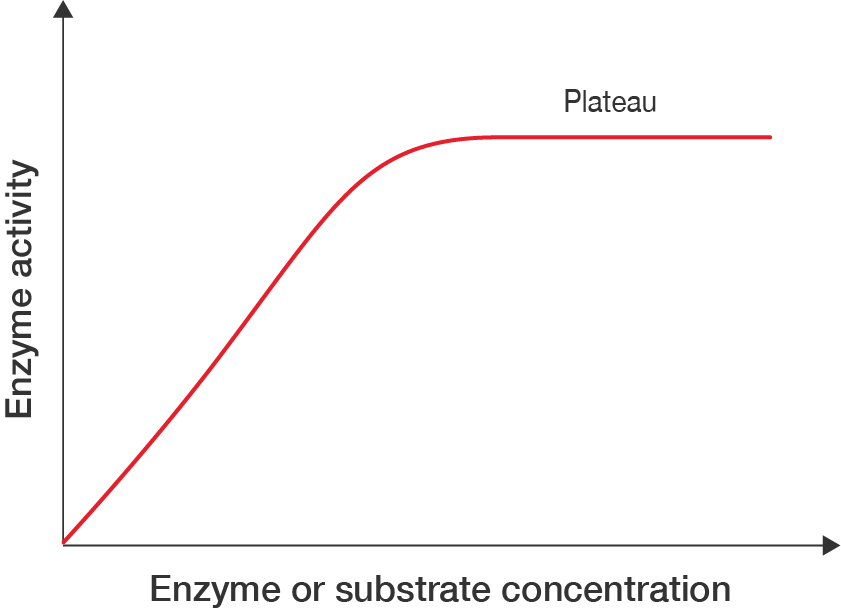
As the concentration of substrate or enzyme increases, the enzyme activity increases… but only until a certain point. If there is a finite amount of substrate and they are all bound to enzymes already, increasing the concentration of enzymes will not further increase enzyme activity. In the same way, if there is a finite amount of enzymes, increasing the concentration of substrates will increase enzyme activity. Activity will reach a plateau.
The presence of inhibitors and activators can decrease or increase enzyme activity, respectively.
There are three types of inhibitors:
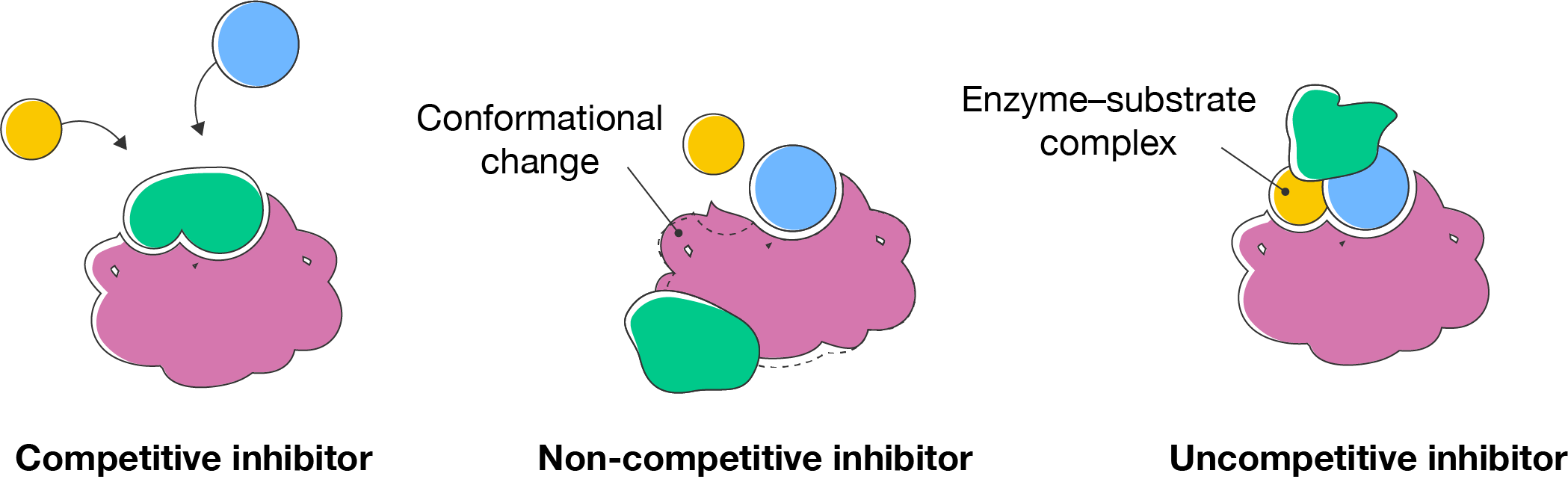
Activators can increase enzyme activity by helping the substrates bind. They might do this by binding to specific sites on the enzyme that help stabilise its structure.
See how well you understand the structure and function of enzymes with a quick quiz.
Images on this page by RMIT, licensed under CC BY-NC 4.0