RNA and protein synthesis
Use this simulation to explore RNA and protein synthesis!
The central dogma of molecular biology describes the flow of genetic material within a biological system. This process is fundamental to understanding how proteins are made using genetic instructions in DNA. Understanding protein synthesis is key to developing therapies in medicine, enhancing crop yields in agriculture and creating new biotechnologies.
The synthesis of proteins is explained using the central dogma (“central dogma” just means it is a fundamental principle or concept). It tells us that DNA is copied into messenger RNA (mRNA) through a process called transcription and then mRNA is used to make proteins in a process called translation.
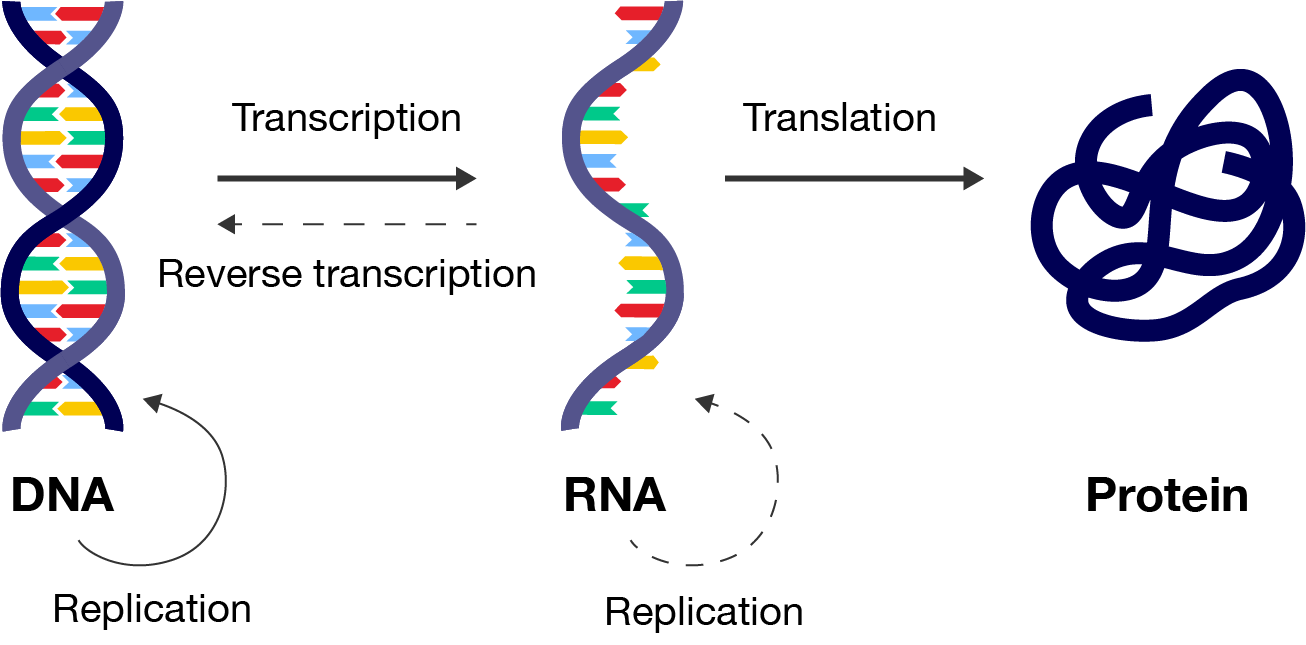
You will soon learn that this process is very complex, with a number of modifications that occur to the mRNA and protein before it is ready for use by the body. Any errors with these processes could lead to a malfunctioning protein.
Transcription (“trans” means “across” and “scribe” means “write”) is the process in which a specific segment of DNA is copied into mRNA. This mRNA strand contains the genetic blueprints for making a protein.
There are three steps to transcribing DNA, which occur in the nucleus of eukaryotic cells: initiation, elongation and termination.
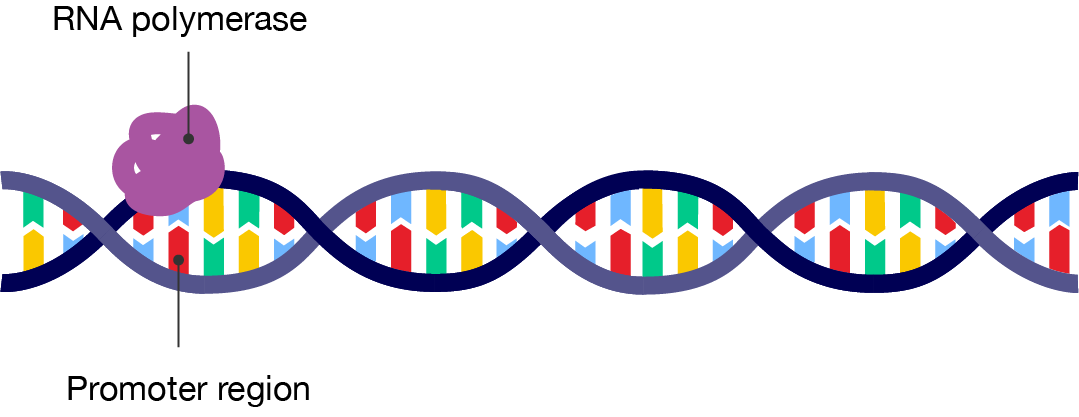
During initiation, an enzyme called RNA polymerase binds to a region of the DNA known as the promoter region. This is the part of a gene that signals the DNA to unwind from its double helix structure, exposing the nucleotides so that they can be read by the enzyme.
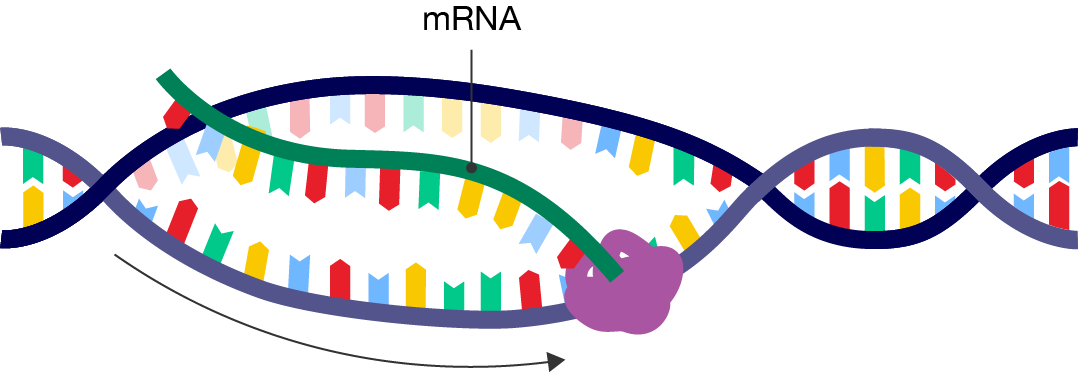
Elongation is when the RNA polymerase moves along the segment of unwound DNA and adds nucleotides in a chain to build the mRNA strand. The mRNA strand is complementary to the DNA strand, following the complementary base pair rule (or Chargaff’s rules).
U and T are similar in structure. The presence of U instead of T indicates that the molecule is RNA, not DNA. Thymine helps keep DNA stable, while uracil is more useful in RNA as it requires less energy to produce.
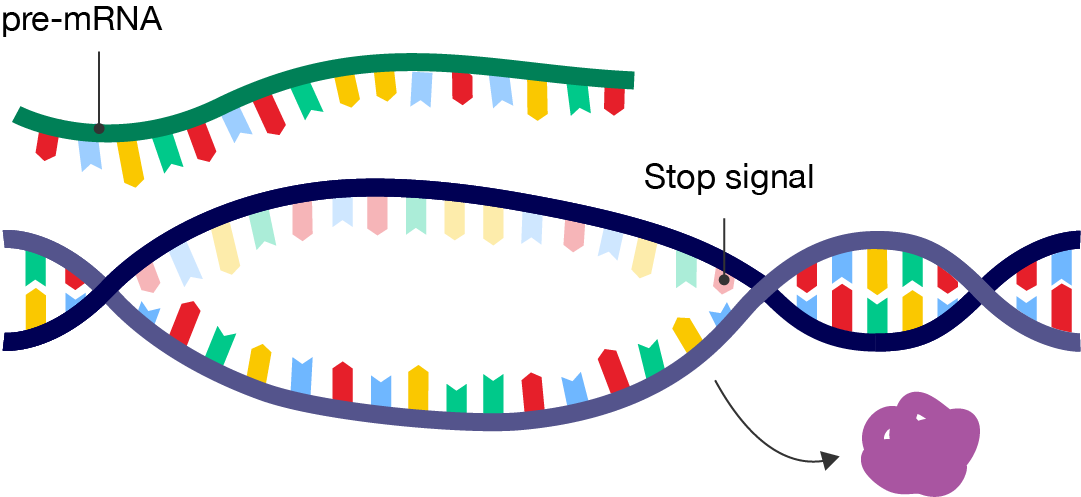
When the RNA polymerase reaches a stop signal (or termination sequence) in the gene, transcription ends. During termination, the RNA polymerase detaches from the DNA, and the mRNA strand is free to carry the blueprints for the protein to a ribosome in the cytosol or endoplasmic reticulum, where it can be translated into a protein.
Watch this video to see transcription in action.
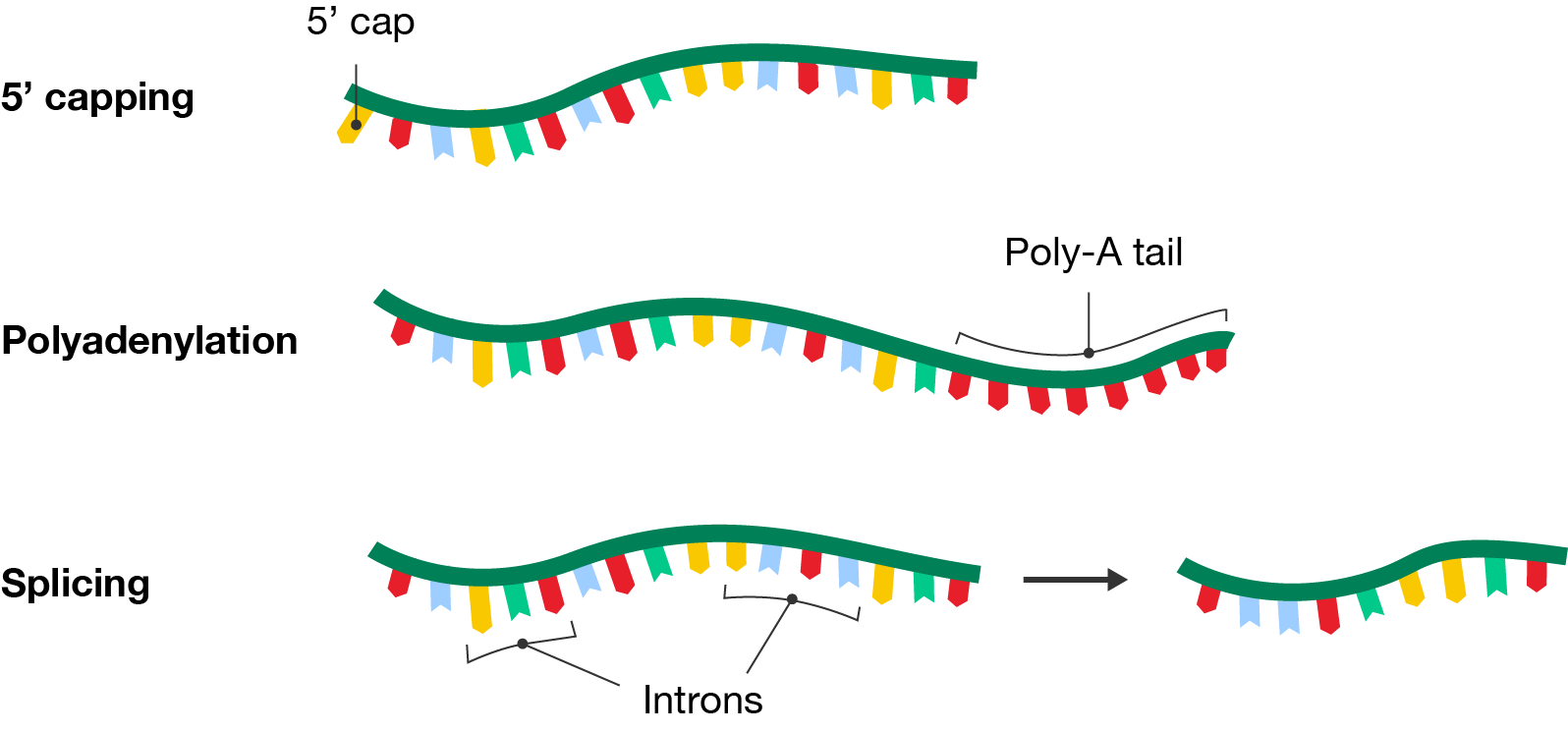
In eukaryotes, transcription forms pre-mRNA which needs to undergo additional modifications like 5’ capping, polyadenylation and splicing to prepare it for translation.
Translation is the conversion of the genetic code from the mRNA into a specific sequence of amino acids, forming a polypeptide or protein. It occurs within ribosomes, which consist of two subunits. The small subunit binds the mRNA transcript and the large subunit binds transfer RNA (tRNA).
Ribosome by MajoraMaster on Sketchfab, licensed under CC BY 4.0
tRNA molecules read the mRNA template in sets of three nucleotides, called codons, with each codon corresponding to a specific amino acid. They bring the appropriate amino acids to the ribosome so they can be added to the polypeptide chain.
The codon chart is shown.
It organises the codons by the three bases that make them up: Each cell within the chart represents a codon made up of a combination of bases, specifying a particular amino acid or a start/stop signal. Here's a breakdown:
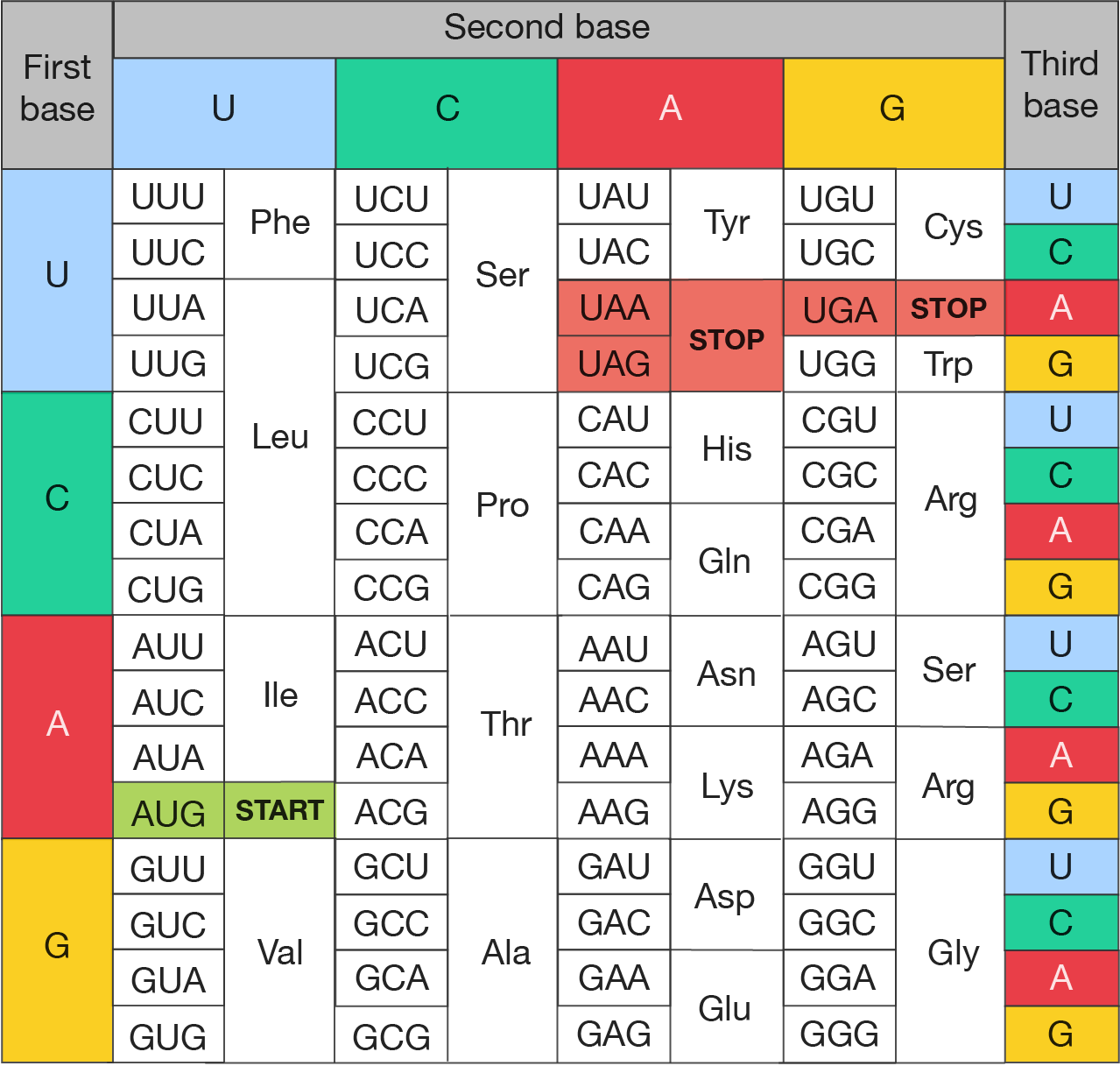
Codon chart
Like transcription, translation involves an initiation, elongation and termination step.
To initiate translation, a complex structure is formed. Proteins called initiation factors help the mRNA template bind with the small subunit of the ribosome and an initiator tRNA, which recognises the start codon, AUG. As well as acting as the initiator of translation, AUG corresponds to the amino acid methionine (Met).
Once the start codon is identified and the small subunit, initiator tRNA and mRNA complex is formed, the next step can occur.
A segment of mRNA with a ribosome and transfer RNA attached. The small subunit of the ribosome is attached to the mRNA at the start codon. The large subunit is attached to an initiator tRNA molecule, which has methionine associated with it. The large subunit has three sites for tRNA to bind. The middle site is occupied by the initiator tRNA. This complex is shown moving towards the small subunit using an arrow.
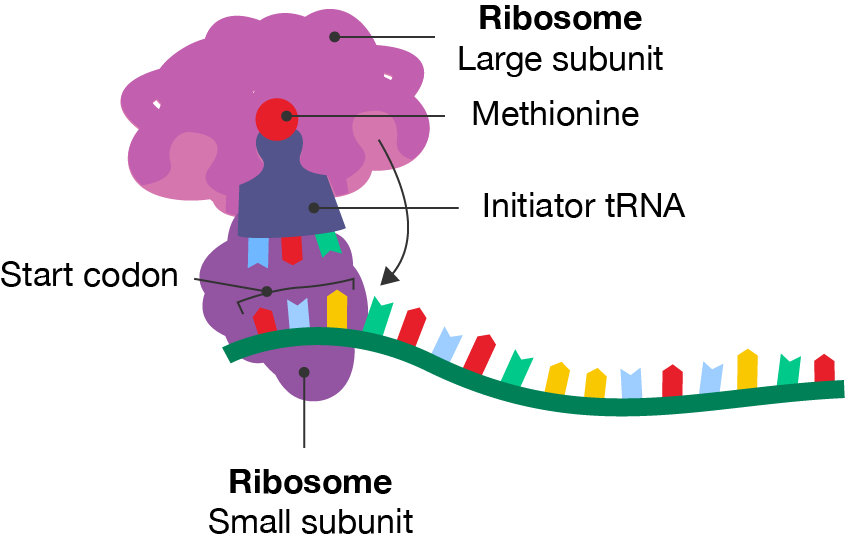
Initiation step of protein translation
The mRNA template is read from the 5’ to 3’ direction and tRNA molecules add amino acids in the correct sequence as the ribosome moves along the mRNA template. Elongation mostly involves the large subunit of the ribosome.
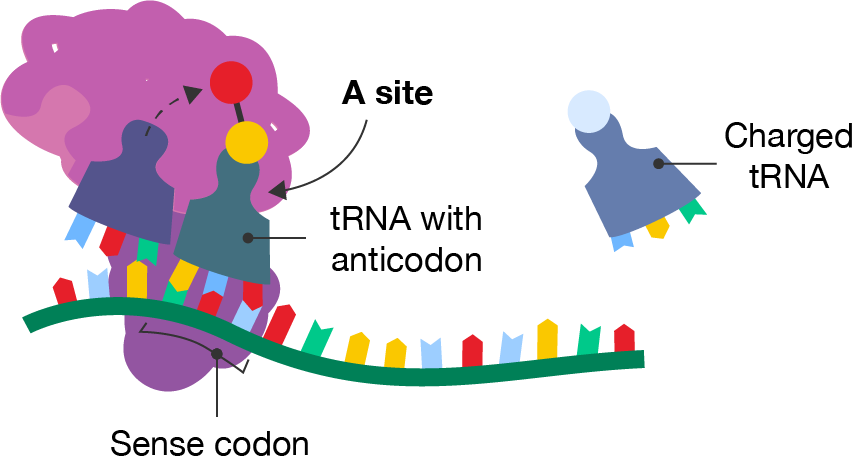
A segment of mRNA with a ribosome and two tRNA molecules occupying the middle and right-hand sites. The methionine is shown attaching to the amino acid on the tRNA in the right-hand site, which is labelled the tRNA with anticodon. The anticodon matches with the codon, labelled the sense codon, on the mRNA strand. The right-hand site is labelled the A site. Freely floating to the right is another tRNA molecule with a different amino acid, labelled the charged tRNA.

A segment of mRNA with a ribosome and three tRNA molecules occupying the left-hand, middle and right-hand sites. The amino acid is shown attaching to the growing polypeptide chain on the tRNA in the right-hand site. Each tRNA has moved one site to the left from the previous diagram. The middle site is labelled the P site.
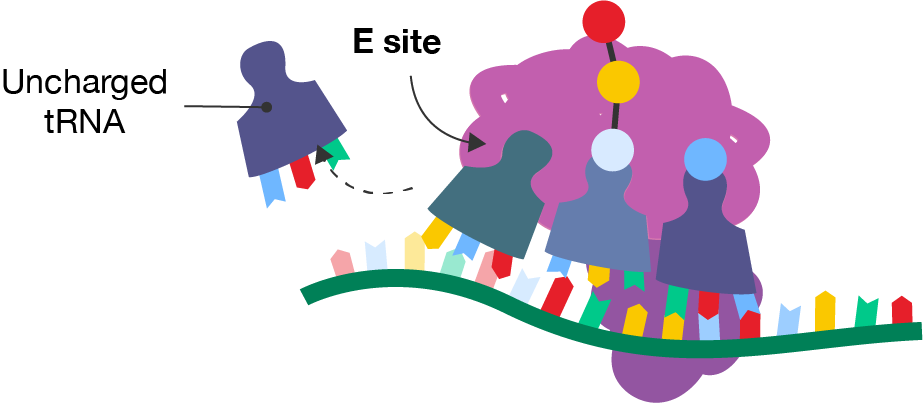
A diagram showing a segment of mRNA with a ribosome and three tRNA molecules occupying the left-hand, middle and right-hand sites. The right-hand site contains a tRNA molecule with a blue amino acid. The middle site contains a tRNA molecule with the rest of the polypeptide chain. The left-hand site is labelled the E site. Each tRNA has moved one site to the left from the previous diagram. Freely floating to the left is the tRNA molecule that was in the E site in the previous diagram. It is labelled the uncharged tRNA.
By freeing up the A site, the elongation process is made more efficient as the next tRNA molecule can add its amino acid.
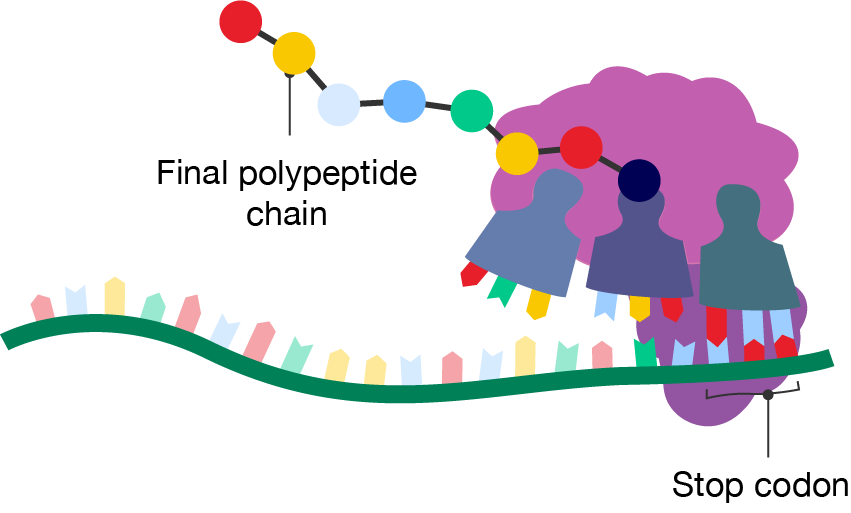
Translation ends when the mRNA presents a nonsense codon or stop codon, which does not have a corresponding amino acid or tRNA molecule. The nonsense codons are UAA, UAG and UGA.
When a nonsense codon reaches the A site, peptidyl transferase adds a water molecule to the carboxyl end of the amino acid. The protein is released from the ribosome and translation is complete. After this stage, the protein has acquired its primary structure.
Watch this video to see translation in action.
Just like after transcription, there are modifications that are often made after translation. This depends on the specific role of the protein, its destination within or outside the cell, and the needs of the cell.
There are many modifications that can be made to alter the protein’s function, activity, stability or location. Common examples are:
These modifications occur in the Golgi apparatus and endoplasmic reticulum. Then, the protein is folded into its secondary, tertiary and sometimes, quarternary structure.
Humans have over 100,000 different proteins. Together with the unique sequence of amino acids, post-translational modifications give proteins their specificity. This accounts for the diversity of proteins in the body.
See how well you understand how proteins are synthesised with a quick quiz.
Images on this page by RMIT, licensed under CC BY-NC 4.0